Researchers Develop a Simplified Method to Modify Disease Signaling with Light
Cellular optogenetics is a technique that allows researchers to use light to precisely control cell signaling and function in space and time enabling the investigation of mechanisms involved in disease processes. A research team at the Turku Bioscience Centre of the University of Turku have developed a novel way to make cellular optogenetic tools much easier to monitor and apply, and showed how they can be used to investigate the cellular side effects of medicines used to treat cancer.
Most diseases are caused by aberrant cell signaling processes and basic research in cell signaling is needed to identify targets for future therapeutic approaches, especially in cases where no cures or effective treatments are currently available.
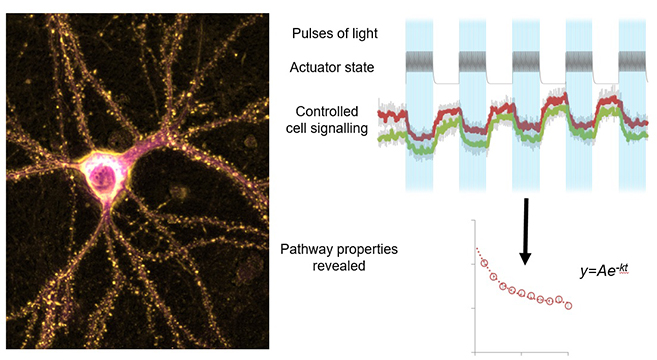
Cellular optogenetics uses light to precisely control cell signaling in space and over time, making it an invaluable technique for disease research. However, this potentially revolutionary method has been difficult for many researchers to use as, over long periods of time, the used light can itself have adverse effects on biological systems and the optogenetic tools can inactivate unexpectedly rapidly.
Now, researchers from the University of Turku in Finland, in collaboration with Frankfurt University Hospital in Germany, have developed a novel way to harness the quantum mechanical phenomenon of resonance energy transfer to design optogenetic tools that are more sensitive to light. The new method also informs the user exactly when an optogenetic tool is going to inactivate in cells. If continued activity is required, just the right amount of additional light can then be re-applied to re-activate the tool.
Combining these advances with existing tools and knowledge, the researchers were able to design and build more efficient optogenetic tools to investigate signaling pathways. With the improved tools, they studied two common chemotherapy drugs known to cause side effects on neurons and cause neuropathic pain. The new tools revealed how both activatory and inhibitory pathways contribute to the actions of these drugs on the investigated disease-associated pathway.
– Now we can develop more powerful tools to understand precisely how harmful conditions disrupt signaling in living cells. This information is likely to help us in identifying targets and designing better therapeutic compounds for conditions such as chemotherapy-induced neuropathic pain, says Lili Li, the lead author of the study and Postdoctoral Researcher at the Turku Bioscience Centre.
– There is still considerable potential to further exploit these quantum mechanical phenomena to devise even better quantitative and informative methods in biology and medicine, which could support the future discovery of new therapeutic approaches, adds senior author of the study Michael Courtney.
This research was funded by the Magnus Ehrnrooths Foundation, the National Institutes of Health’s National Cancer Institute (grantome.com/grant/NIH/R01-CA200417), the European Union Erasmus + programme and 7th Framework Programme Initial Training Networks, the Academy of Finland and DAAD mobility programmes and it was supported by facilities of the Turku Screening Unit, a member of the Biocentre Finland Drug Discovery and Chemical Biology Network.
The study was published in the journal Nature Communications.
Research article: Resonance energy transfer sensitises and monitors in situ switching of LOV2-based optogenetic actuators
More information: Senior Research Fellow Michael Courtney, Turku Bioscience Centre, University of Turku, +358 50 464 9827, michael.courtney@bioscience.fi
