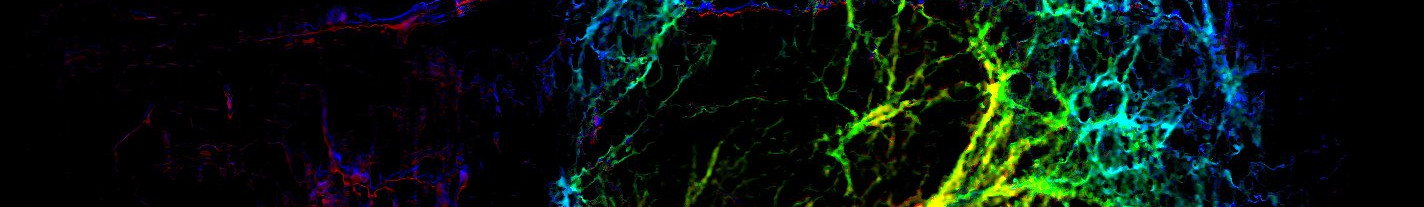
Intermediate filaments (IFs) form structures that are essential for providing mechanical strength to cells. Interestingly, recent data point to an important function of IFs in cell signaling as scaffolding of critical signaling molecules. Our lab focuses on studying the signaling-mediated regulation of vimentin IFs as well as the role of IFs as signaling modulators in diverse physiological phenomenon. Vimentin at the interface of cell growth and autophagy Handled by Ponnuswamy Mohanasundaram & Leila Rato Type III intermediate filament protein vimentin, abundantly expressed in mesenchymal cells is one of the well-known marker for epithelial to mesenchymal transition (EMT). EMT occurs during embryonic development, cancer and wound healing. During EMT, cell undergo morphological changes from epithelial to mesenchymal and acquire stem cells-like properties such as highly proliferative, migratory and invasion. Our current research is focused on vimentin’s role in physiological and pathological conditions, using both in vitro and in vivo models. We found that vimentin plays an important role in wound healing mechanism, by regulating EMT, cell proliferation, extracellular matrix production, growth signaling and migration. Vimentin is significant for directional persistence of migrating cells Handled by Arun Venu & Mayank Modi Directional persistence is essential for the successful wound healing since the cells on the wound bed should migrate to their target destinations in response to the signaling cues from platelets and macrophages after the injury happens. The extracellular matrix deposition and further remodeling to fill the wound gap depends on proper directional migration of fibroblasts and epithelial cells. Vimentin is found to be crucial for cell migration. Vimentin deficient mice showed impaired wound healing. The fibroblasts isolated from vimentin deficient cells have been shown to lack directionality during migration. Vimentin in ECM deposition, organization, and remodelling Handled by Mayank Modi Vimentin mediated intrinsic polarity, size, and morphology of fibroblasts has a direct influence on the deposition of ECM during wound healing. Vimentin regulates not only the synthesis of different ECM components such as collagen type 1 and fibronectin but also dictates the organization of ECM in the form of anisotropy, alignment, structure and patterning. These properties can be influenced by mechanical properties of the substrate. Additionally, intrinsic polarity of fibroblasts in the presence of vimentin which propels migration directionality effects the anisotropy and patterning of ECM during the ECM remodelling (resolution) phase of wound healing. We study the mechanosensory role of vimentin in wound healing homeostasis through fibroblasts at the intersection of traditional laboratory techniques and advanced microscopy techniques such as multiphoton and confocal microscopy, super resolution microscopy, and traction force microscopy. Vimentin in fat metabolism Handled by Peiru Yang Adipocytes maintain energy homeostasis and metabolism. During adipocyte differentiation, lipid synthesis occurs and excess energy is stored in lipid droplets (LDs). Electron microscopy studies revealed that in the process of adipocyte differentiation, Lipid droplet development is accompanied by complete structure rearrangement of vimentin intermediate filaments (IFs). Vimentin IFs undergo a dramatic reorganization changing from a dispersed network to fully developed cage complex which anchored firmly on the surface of LDs. Proteomic profiling of LD-associated proteins from obese mice also showed down-regulation of vimentin. The potential role and the identification of the specificity of vimentin IFs with LD development and lipid metabolism will provide a new insight in understanding obesity and type II diabetes. Vimentin and nuclear integrity Handled by Emilia Holm It is known that migratory cells, such as mesenchymal cells, express the intermediate filament vimentin. In these cells, vimentin often form a “cage” around the nucleus, with filaments extending towards the cellular periphery. Recent research has shown that the vimentin cage can provide mechanical support to protect the nucleus and genome during migration. It is, however, not known whether the vimentin cage also is involved in maintaining the integrity of the nucleus and its components in other situations, such as stress situations. In this project, we are looking to address the question of what role vimentin has in the maintenance of nuclear integrity, by exposing the cells to for example heat stress. The role of vimentin in resorbing osteoclasts Handled by Elnaz Fazeli We study vimentin interaction with other cytoskeletal elements in resorbing osteoclasts derived from mice. To enable the study of resorption in conjugation with cytoskeletal dynamics, we use bone-mimicking substrate developed in the house employing 20nm bone particles as the main element of the substrate. Cells are cultured on this bone mimicking surface, differentiated, and polarized osteoclasts containing multiple nuclei resorb the organic and inorganic elements of the bone-mimicking substrate. At this stage, live cell imaging is performed using state of art imaging techniques such as spinning disk confocal and TIRF microscopes. Moreover, we develop image analysis workflows in order to analyze big datasets of live cell imaging data automatically. The role of exosomal vimentin in mediating wound healing Handled by Sepideh Parvanian Despite a great progress made during last decades to improve cutaneous wound healing, treatments are challenging and often ineffective. Accumulating evidence suggest that extracellular vesicles (EVs) derived from mesenchymal stem/stromal cells (MSC) are promising alternatives to stem cells in regenerative therapy. As one of the EV subsets, exosomes with the size of 30-150 nm in diameter have potential to promote tissue repair, due to their intrinsic features such as high stability, non-immune rejection, homing effect, easy control of dosage and concentration. Our lab previously showed that vimentin is involved in wound healing process by controlling fibroblast proliferation, TGF-β1–Slug signaling, collagen accumulation and epithelial–mesenchymal transition (EMT) processing, a process that occurs during development, wound healing, and cancer metastasis and loss of vimentin can lead to a severe deficiency in fibroblast growth. Interestingly, vimentin have been reported detectable in exosomes from different cell types. During my PhD project, we reveale the active and necessary role of exosomal vimentin from adipocyte progenitors in promoting fibroblast proliferation, migration, and ECM secretion. Our results from in vivo and in vitro experiments present strong evidence that adipose progenitor- exosomal vimentin has a critical role in shortening the healing time and reduce scar formation. These findings suggest a novel role for exosomes in mediating wound repair by transferring cytoskeletal proteins.Intermediate filaments as signaling determinants
Funding: Academy of Finland and Sigrid Jusélius Foundation
Cell proliferation and growth are two coordinated processes, essential for normal development and body homeostasis. These processes influence developments, wound healing and cancer. We have found that vimentin regulate cell size through the insulin/Akt/mTOR signaling by modulating nutrient and growth signals, along with that it also regulate autophagy through the same signaling axis. Currently, we are trying to decipher the molecular mechanism of vimentin in cell growth and autophagy.
Furthermore, we are interested in understanding how different nutrient availabilities affect mTORC1 signalling in the wildtype and vimentin deficient cell models and how it affects cell growth. Furthermore, we want to recognize the signalling partners involved in this process, as well as changes in the phosphorylation status by utilizing state of the art techniques such as mass spectrometry.
Focal adhesions (FAs) are macromolecular assemblies that help cells to attach to the extracellular matrix which facilitate inside-out and outside-in signaling transduction. These signals include both biochemical and biomechanical signals from the ECM and to the ECM via actin- integrin links. Integrin adhesome is highly complex network of the protein networks involved in cell migration, cell cycle, cell differentiation and apoptosis. Intermediate filaments (IFs) have been well established as key interactors with cell junction complexes but they have not been described to interact with FAs although, taking into account their roles in cell and tissue integration, it would be rational to assume the IF-engagement with FAs. In conjunction with studies on key roles of vimentin IFs in wound healing, we have reported a bidirectional interaction of vimentin IFs with microfilaments. Further examination revealed the association of vimentin with FAs. Therefore, our project aims to find out the nanoscale positioning of vimentin in FAs in association with other protein components of FAs. We use super resolution microscopy modalities such as STED, PALM, dSTORM to resolve the nanoscale arrangement of vimentin in FAs, which is a key aspect in terms of resolving the focal adhesion nanostructure.
Human Papilloma Virus (HPV) associated cancers & diseases
Targeting Human Papilloma Virus-positive cancers with anisomelic acid
There is a high global burden of Human Papilloma Virus (HPV) driven cancers. Despite HPV vaccinations, HPV-driven cervical cancer is the second cause of cancer deaths in women worldwide. Equally alarming, HPV is behind a veritable epidemic of other epithelial cancers, including oral, and head & neck cancers, which are steadily on the rise. On top of that HPVs are also causing various types of warts or papillomas of skin and anogenital tract. Currently there are no specific treatments for patients with HPV-driven cancers. Although HPV vaccines yield promising effects, there is globally an acute, extensive, and unresolved need for effective and targeted anti-HPV treatments in cervical, anogenital, head & neck, and oropharyngeal cancers.
Our research team has found a novel way to eliminate HPV-infected cells and thus prevent the development of diseases caused by HPV. In a pioneering study, we showed that a naturally occurring compound Anisomelic Acid (AA), as well as its synthetic derivatives that we have developed based on this compound, effectively degrade the HPV E6 and E7 oncoproteins, i.e., the carcinogenic proteins in the virus that are known to play a key role in promoting the development of HPV-positive cancers. Our research focuses on understanding the degradation pathway of E6 and E7 in HPV-positive cancer cells upon the treatment with those anti-HPV compounds.
The molecular mechanisms of downregulation of E6/E7 oncoproteins
Our previous results shows that both the E6/E7 oncoproteins are subjected to protein degradation in response to ansisomelic acid (AA). Currently, we are studying how this degradation is triggered by activation of a degradation machinery based on either shared or unique components. We are using Tandem Ubiquitin Binding Entity (TUBE) technology that purifies and identifies the ubiquitinated proteins and UbiQuantTM to quantify the ubiquitination of the E6/E7 oncoproteins, followed by identification of the ubiquitination sites with advanced mass spectrometry. The goal is to identify specific E3 ubiquitin ligases that direct the target proteins to the proteasomal degradation machinery. This information will be necessary for developing our targeting strategy to eliminate HPV-driven cancers.
Binding of Anisomelic Acid to E6 and E7 oncoproteins
AA-dependent depletion of E6 and E7 induces apoptosis and efficiently inhibits cell growth. However, we are yet to understand the possible interactions of AA with these proteins. To unravel this, we are currently using various techniques like Cellular Thermal Shift Assay (CETSA), Thermal Shift Assay (TSA) and microscale thermophoresis technology to prove the interaction of AA with E6 and E7.
Targeting HPV lesions In a Transgenic Mouse Model
Our research groups’ earlier in vitro studies, demonstrated that the AA, a diterpenoid from plant Anisomeles Malabarica, leads to degradation of the viral oncoproteins, E6 and E7. According to the promising results from in vitro studies, we are proceeding to establish proof of concept in vivo in a HPV mice model. In this regard, we have chosen K14-HPV16 transgenic mice, which have been validated as a model of squamous cell carcinoma. In this strain, the expression of early region of HPV16 gene, including E6 and E7 is under the control of human keratin-14 promoter/enhancer which eventually creates dysplastic lesions on the skin of the mice. Prime objectives of this project are to test the efficacy of AA and AA derived small molecules in vivo on K14-HPV16 transgenic mice lesions both via topical and other routes of administration.
HPV Horizon Project
In this project, funded by the Jane and Aatos Erkko Foundation, we use our previous findings to develop an AA/small molecule-based combination therapy that specifically eliminates the cancer-promoting mechanisms. The main goal of the project is to find new therapeutic measures to specifically eliminate tumors caused by HPV infection. Our research is focused on establishing a novel dual-targeting therapeutic strategy that targets both the unique E6 and E7 -dependent features and the general stress protection machinery to eliminate HPV-driven cancers.
More details about this project can be found at HPV Horizon.
Warts & HPV
Warts are the most common dermatological benign lesions caused by HPV in the outer layer of the skin (epidermis). Most people develop cutaneous warts at some point in their lives. Our research team under the Business Finland (Business to Research) funding developed a targeted natural medication to get rid of the warts. This has led to the establishment of a start-up, Anison Therapeutics, which is dedicated on developing the product through regulatory pathway for market approval.
You can read more about the start-up at Anison Therapeutics.
Responsible Team Members
- Michael Silva
- Navid Delshad
- Tatiana Tarkhova
- Preethy Paul
- Silvia Gramolelli
Other projects
cFLIP mediated signal transduction in receptor-mediated apoptosis and cell survival
The cellular FLICE-inhibitory protein (cFLIP) is a key regulator of the death receptor apoptotic pathway. The protein has three known isoforms, cFLIP long (cFLIPL), short (cFLIPS) and Raji (cFLIPR). While the short isoform has a distinct role of inhibiting death receptor apoptosis, cFLIPL exhibits a dichotomy in its function between promoting cell death and activating survival pathways. Understanding the mechanisms that serve to determine this crosstalk which results in different cell fates is the central theme of our research.
Regulation of cFLIP by post-translational modifications
The activity of cFLIP, like most cellular proteins, can be regulated at the protein level by post-translational modifications (PTMs). We previously demonstrated that stability of the short isoforms is regulated by classical PKC-mediated phosphorylation on a serine in the prodomain of the protein.
Ongoing research focuses on pinpointing the cFLIPL isoform-specific relevance of two phosphorylation sites we have previously identified. Current findings reiterate the predominantly nuclear localization of cFLIPL and provide evidence of a phosphorylation-dependent subcellular redistribution of the protein in response to signals originating from activated death receptors.
Furthermore, we aim to investigate the relevance of this finding within the context of minimising resistance and improving sensitivity to TRAIL.
cFLIP regulation of cell population size
Our recent findings show that the cFLIP phosphorylation on certain amino acid residues is increased during mitosis, and that the phosphorylated pool of cFLIP proteins is accumulated at the centrosome throughout the cell cycle. In addition, we observe that overexpression of wild type cFLIPL significantly increases the cell population size, whereas overexpression of certain phospho-deficient mutants of cFLIPL causes a decrease in cell population.
Moreover using a pseudo in vivo system (chicken egg model) increased tumour growth is observed in cFLIPL overexpressed cells. These results indicate that by regulating the levels and phosphorylation status of cFLIPL, it is possible to control tumour growth.
We are currently investigating the signalling pathway behind this observation as well as the possible involvement of cell cycle.
Modeling dead and alive: in silico model of cell fate
The research here focuses on iteratively combining mathematical modelling and experiments to understand the role of cFLIP phosphorylation in cell fate decisions.
Our current modelling tasks involve the construction of a single cell and cell population based models to investigate and analyse the dynamics of isoform-specific cFLIP phosphorylation in receptor-mediated cell death signaling and cell cycle progression.
Other related research involves application of systems identification methods for model parameter estimation from flow cytometry data.
The work is done in collaboration with the research groups of Prof Henrik Saxen (Dept of Chemical Engineering ) & Prof H. Toivonen (Information Technology) at ÅÅU.
Responsible Team Members
- Alia Joko
- Erik Nimela
- Preethy Paul
- Michael Silva
Targeting Human Papilloma Virus-positive cancers with anisomelic acid
Human papilloma virus (HPV) is the leading cause of cervical cancer, other epithelial cancers, including oral cancer, various head and neck cancers and other diseases like common and genital warts. HPV-positive cancers cause ~1 million deaths annually, and every year ~1.5 million new diagnoses are being made. There are currently no specific treatments for patients with HPV-driven cancers. We have discovered a plant-derived compound, called anisomelic acid, and synthetic small molecules that specifically target E6 & E7 oncoproteins, the root cause of HPV-associated diseases. We have also developed a largescale production procedure of the active natural compounds for the treatment of HPV-mediated warts. Nowadays, the main goal of our team is to understand the mechanism of action of anisomelic acid. Therefore, we have different projects that assess how anisomelic acid targets E6 and E7 oncoproteins:
- Anisomelic acid mediated E6 and E7 degradation
- E6 and E7 interactome upon AA treatment
- Binding of anisomelic acid to E6 and E7 oncoproteins
Responsible persons
- Senthilkumar Rajendran
- Preethy Paul
- Michael Santos Silva
Sensitization of prostate cancer cells to TRAIL-induced cell death
Prostate cancer cells frequently develop resistance toward androgen-deprivation and chemotherapy. Prostate cancer is the most common malignancy and the second leading cause of cancer mortality in men. Prostate tumors express frequently TRAIL receptors on the cell surface, but these receptors are often not able to trigger TRAIL-induced apoptosis because of increased pro-survival signaling. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been shown to be selectively pro-apoptotic in cancer cells, with minimal toxicity to normal tissues. Although this feature makes TRAIL a promising anticancer agent, not all cancer cell types are sensitive to TRAIL-induced apoptosis despite abundant expression of TRAIL receptors. Thus, combinatorial treatments to sensitize tumor cells to TRAIL-induced apoptosis have been in the focus of extensive research. Dietary lignans have shown cancer preventive and antitumorigenic activity, but the mechanisms behind these effects are poorly known. We have identified few plant lignans that can sensitize prostate cancer cells to TRAIL-mediated apoptosis. We have also to certain extent characterized the key structural features for their function and defined their mechanism of action.
Responsible persons
- Preethy Paul
Nanoparticles as carriers of antitumor drugs
To be able to target cells of interest is one of the key aims in medicine today, because it could minimize side effects of drugs. This is of special importance in cancer therapies, were the treatment has serious side-effects on healthy cells. Nanomedicine seeks to overcome this limitation by utilizing nanoparticles as targeted drug carrier systems. Together with Jessica Rosenholm at the Pharmaceutical Sciences Laboratory at Åbo Akademi University and Cecilia Sahlgren at the Cell Fate Laboratory at Turku Centre for Biotechnology, we have shown that specific targeting of nanoparticles to cancer cells is possible and, furthermore, that the nanoparticles can be used as
carriers for antitumor drugs with minimal off targeting effect. The collaboration aims at developing this concept further in order to get better specificity, and also to employ the particles as carriers for
other types of compounds such as anti-inflammatory drugs that could be beneficial e.g. ineurodegenerative diseases.
Responsible person
- Erik Niemelä
Functionnal materials at Biological interfaces
Functional Materials at Biological Interfaces (FunMat-consortium) aims to develop innovative materials and functionalized surfaces for the control and sense of cell functions. By temporal and spatial control of cell function we will be able to design novel biological structures to investigate fundamental aspects and applications of tissue engineering and regeneration therapies, tissue monitoring, biotechnology, biomaterials design, drug screening and development.
Responsible persons
- Hend Abdelkader
- Arun Venu
- Emilia Holm
- Mayank Modi